SARS-CoV-2 Antiviral VV116
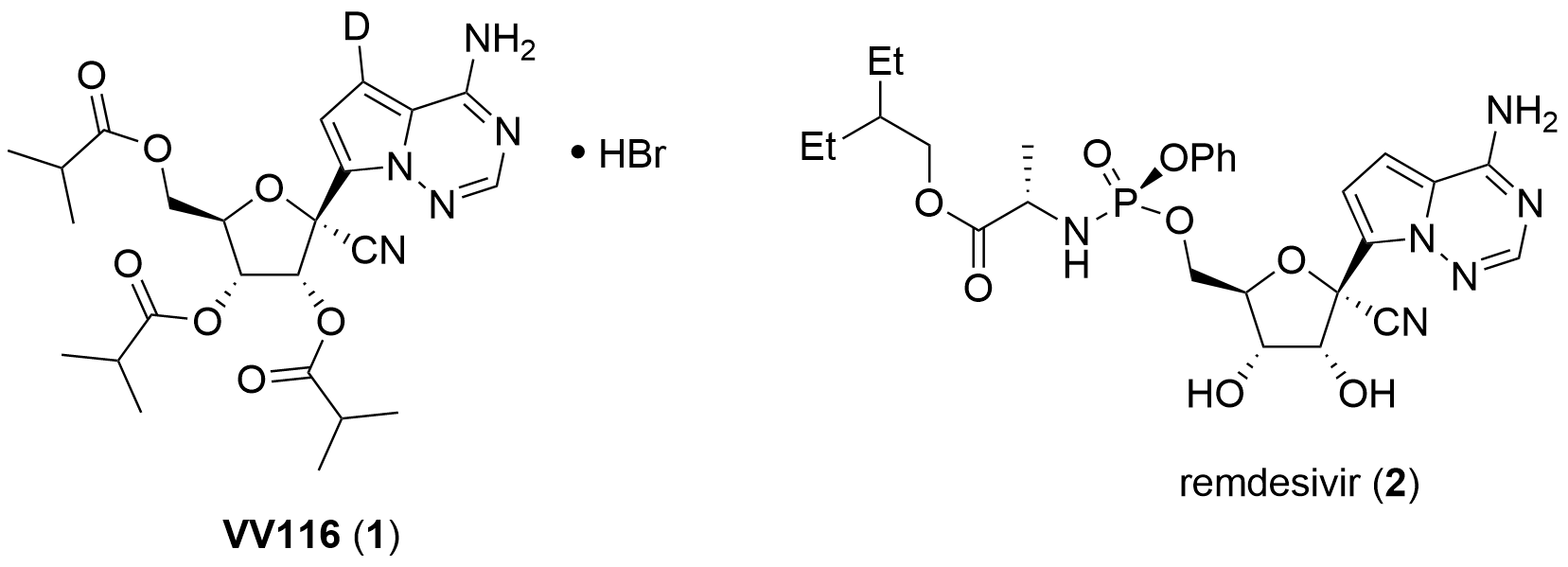
The nucleoside analogue VV116 (1, Fig. 1) is a nucleoside analogue derived from remdesivir (2) showing antiviral activity against SARS-CoV-2 first described by Leike Zhang, H. Eric Xu, Jingshan Shen and co-workers from the Shanghai Institute of Materia Medica and the University of Chinese Academy of Science Beijing. In their initial report published in a letter to Cell Research [1] and an article in Bioorganic & Medicinal Chemistry[2] they claimed a higher bioavailability for compound 1 compared to 2 which had to be intravenously administered.
Figure 1: Structure of VV116 (1) and remdesivir (2).
Synthesis of VV116
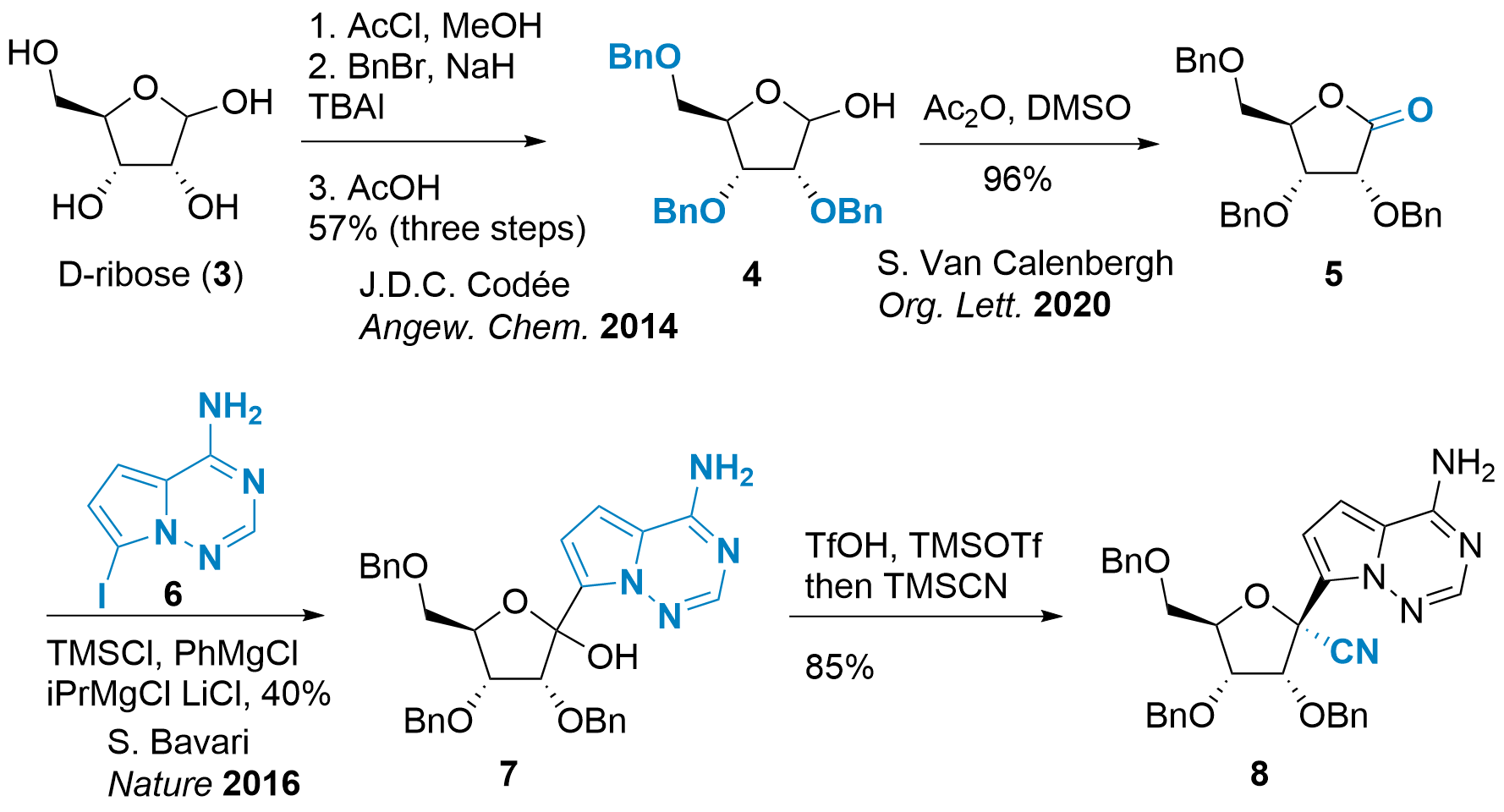
The nucleoside analogue can be synthesized in several steps from ribose (3) as shown in Scheme 1 and 2. However, some intermediates like 5 may be commercially available. Jeroen Codée described in 2014 [3] the synthesis of protected ribose 4 in 3 steps, then Albright-Goldman oxidation gave access to ester 5 [4] as shown in Scheme 1. The following steps were first described by Thomas Chilar and Sina Bavari in Nature 2016.[5] Activation of iodine 6 allowed the addition to 5 yielding 7 in poor yield. Finally, activation of the alcohol was followed by the substitution with the cyanide in a diastereoselective fashion to 8; a common precursor for ribavirin (2, not shown) and VV116 (1).
Scheme 1: Synthesis of the common precursor 8 towards antiviral VV116 (1).
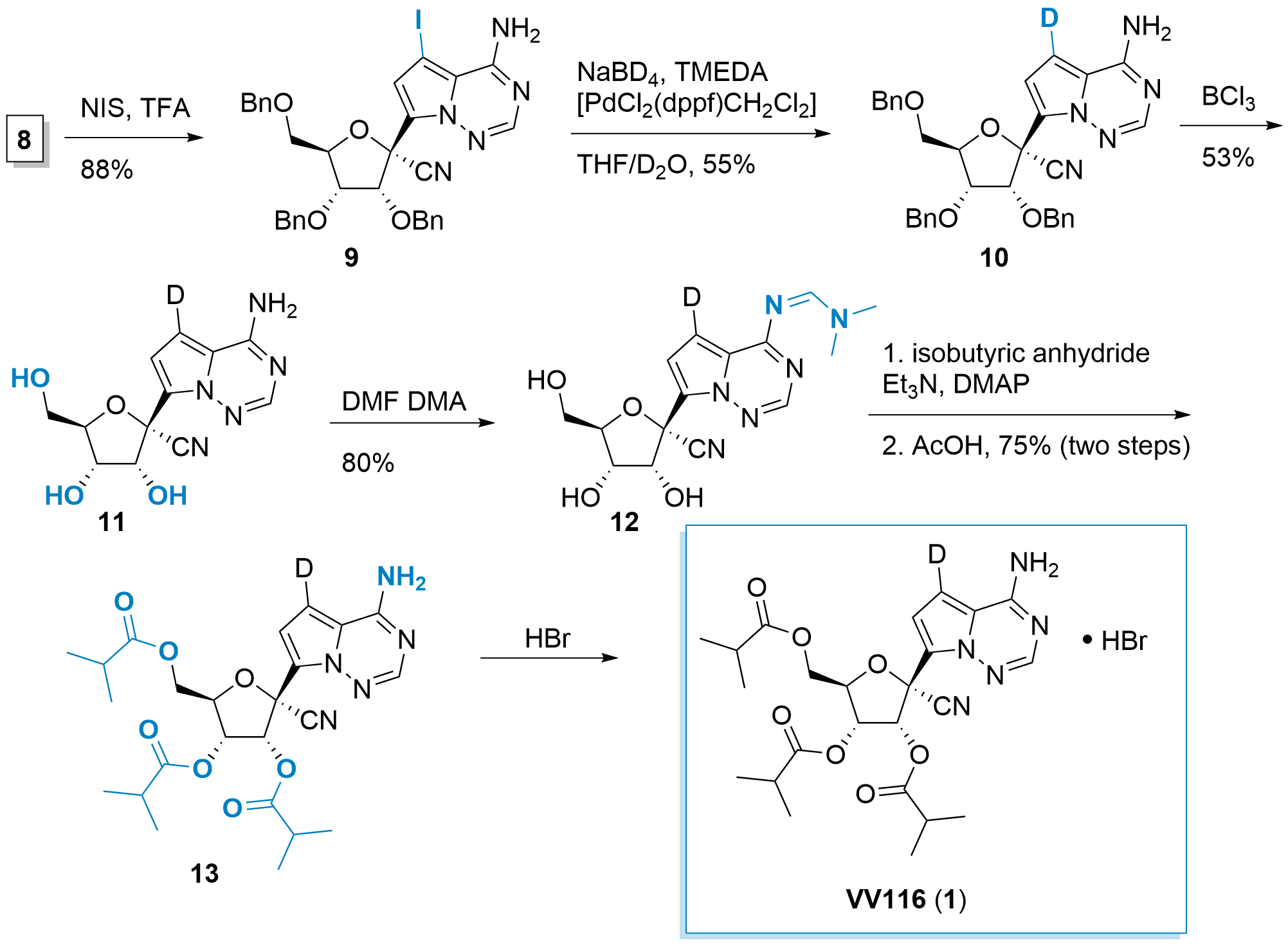
With 8 in hand, Jingshan Shen and co-workers reported the synthesis of VV116 (1) as shown in Scheme 2. Iodination allowed the activation of the purine analogue in the C-7 position. This was followed by the palladium-catalyzed deuterium introduction to 10. Then, Lewis-acidic deprotection gave 11. Before the ester formation, the primary amine has to be protected and therefore 12 was synthesized. Now, common conditions gave and subsequent deprotection gave 13 in good yield. Finally, 13 was converted into the HBr salt 1.
Scheme 2: Finalization of the synthesis of VV116 (1).
Antiviral Activity
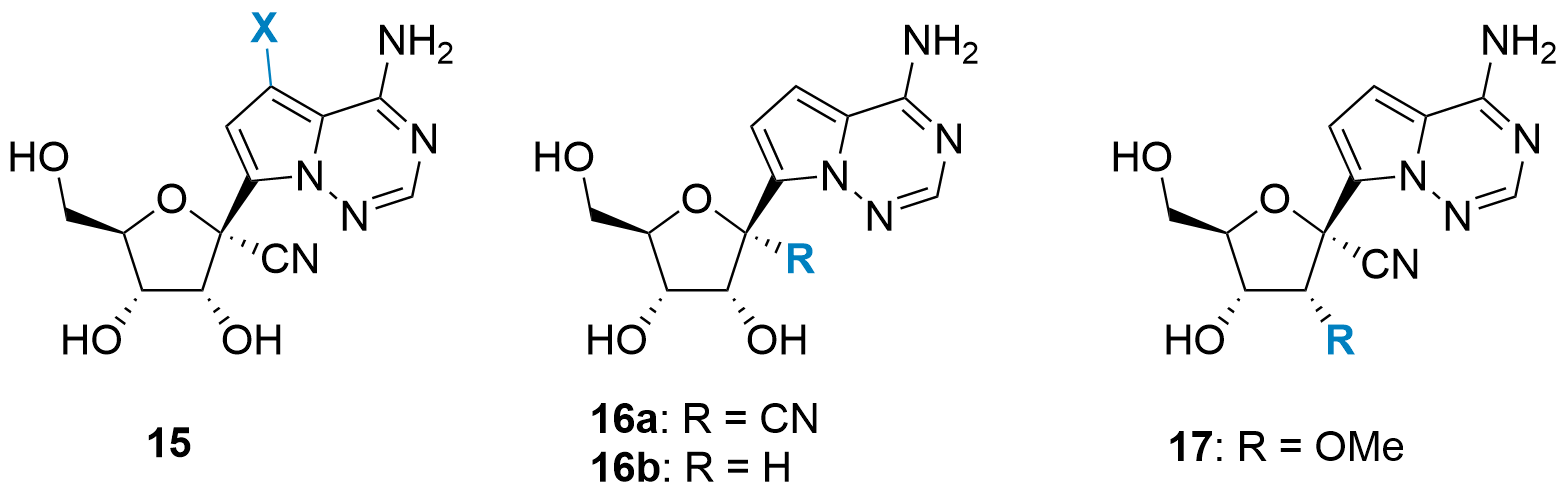
Initial SAR studies
| compound |
antiviral assessment |
EC50 |
CC50 |
| VV116 (1) | |||
| 15a (X = F) |
moderate activity |
3.30 µM |
n.d. |
| 15b (X = Br, Cl, I, OH, CN) |
no activity |
n.d. |
n.d. |
| 15c (X = D) |
highly active |
0.39 µM |
> 500 µM |
| 16a |
0.59 µM | > 500 µM | |
| 16b |
highly cytotoxicity |
n.d. |
n.d. |
| 17 |
no activity |
n.d. |
n.d. |
Table 1: Initial SAR study towards VV116 (1).
Then different esterification patterns were probed. As ester moieties often get cleaved after admission, their main purpose is to change their solubility and cell permeability properties. Therefore, esterification of 15c led to several nucleoside analogues with similar antiviral activity, however, with changed bioavailability. In the end, VV116 (1) showed the best properties concerning bioavailability (F = 80%) in comparison to 15c (F = 22%).[1]
Phase 3 Trial (R. Zhao, 2022)
Ren Zhao and colleagues from the Shanghai Jiao Tong University (Shanghai, China) reported phase 3 results in the New England Journal of Medicine in late 2022.[6] Their study had a total of 822 patients during a local SARS-CoV-2 outbreak in early 2022 (omicron), around half of them receiving VV116 (1) whereas the other half received Nirmatrelvir–Ritonavir (paxlovid) as a treatment. Their results indicated, that the treatment with 1 was not inferior to paxlovid and no patient in both group had progressed to severe conditions. Furthermore, patients receiving 1 reported fewer adverse events compared to paxlovid.
Literature
[1] Y. Xie, W. Yin, Y. Zhang, et al. Design and development of an oral remdesivir derivative VV116 against SARS-CoV-2. Cell Res 2021, 31, 1212–1214. https://doi.org/10.1038/s41422-021-00570-1
[2] D. Wei, T. Hu, Y. Zhang, W. Zheng, H. Xue, J. Shen, Y. Xie, H. A. Aisa. Potency and pharmacokinetics of GS-441524 derivatives against SARS-CoV-2, Bioorg Med Chem 2021, 46, 116364. https://doi.org/10.1016/j.bmc.2021.116364
[3] E.R. van Rijssel, P. van Delft, G. Lodder, H.S. Overkleeft, G.A. van der Marel, D.V. Filippov, J. D. C. Codée Furanosyl Oxocarbenium Ion Stability and Stereoselectivity Angew Chem Int Ed 2014, 53, 39, 10381-10385. https://doi.org/10.1002/anie.201405477
[4] J. Bouton, S. Van Calenbergh, J. Hullaert Sydnone Ribosides as a Platform for the Synthesis of Pyrazole C-Nucleosides: A Unified Synthesis of Formycin B and Pyrazofurin Org Lett 2020, 22, 23, 9287-9291. https://doi.org/10.1021/acs.orglett.0c03523
[5] T. Warren, R. Jordan, M. Lo, et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 2016, 531, 381–385. https://doi.org/10.1038/nature17180
[6] Z. Cao, W. Gao, H. Bao, et al. VV116 versus Nirmatrelvir–Ritonavir for Oral Treatment of Covid-19 New Engl J Med 2022. https://doi.org/10.1056/NEJMoa2208822