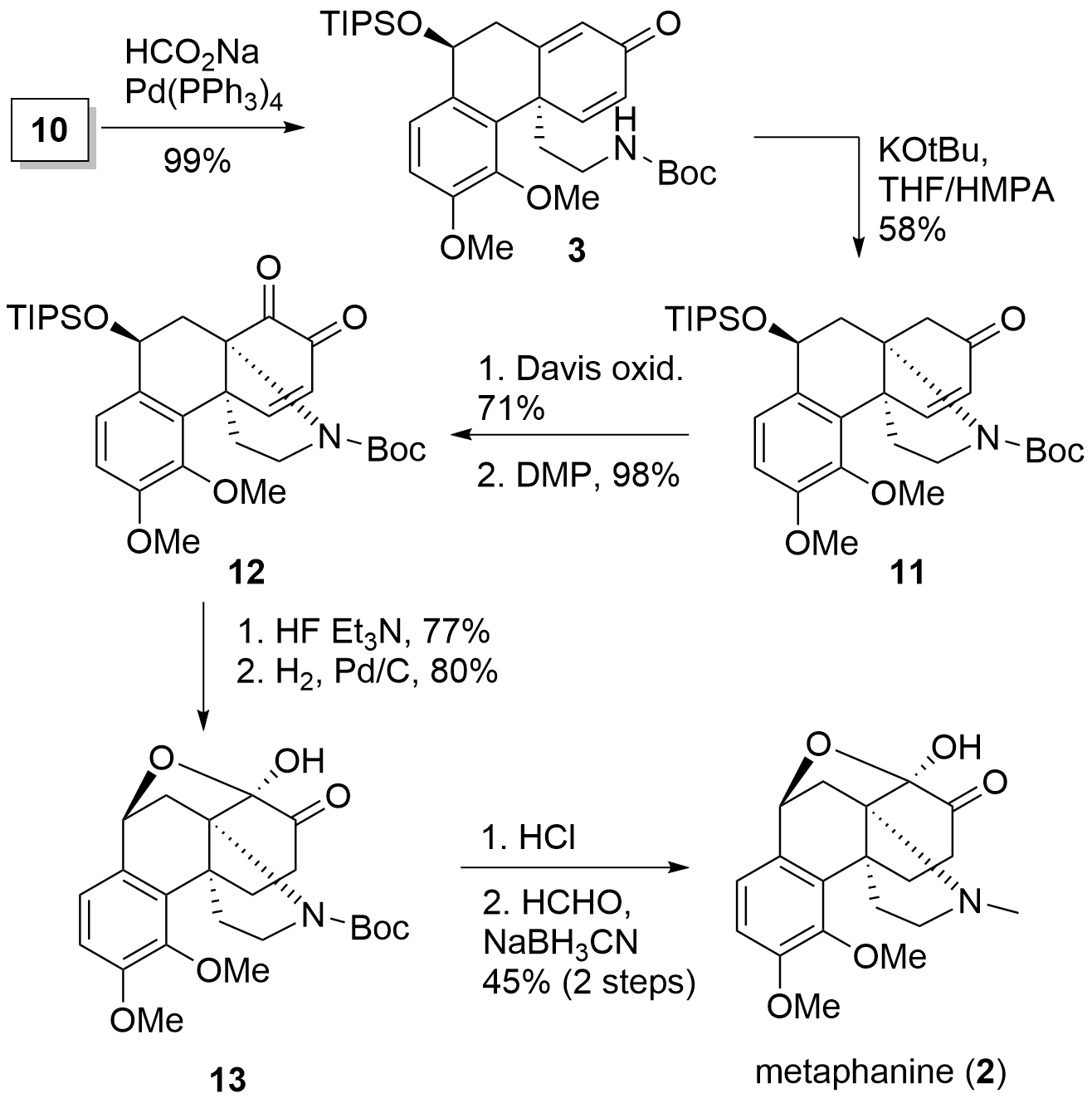
Total Synthesis of Stephadiamine and Metaphanine (K. Nagasawa, 2021)
Stephadiamine (1, see Figure 1) and metaphanine (2) are two alkaloids with a hasubanan core structure. Almost 40 years after the isolation of stephadiamine (1) the Nagasawa group from Tokyo University of Agriculture and Technology was able to describe a synthetic approach to this natural product in the journal JACS. They hypothesized that stephadiamine could be biosynthetically available from metaphanine (2) through rearrangement and therefore opted for a late-stage biomimetic transformation. Metaphanine (2) itself should be available from unsaturated system 3 by aza Michael addition and oxidation. For the synthesis of 3, an intramolecular oxidative coupling of 4 was envisioned.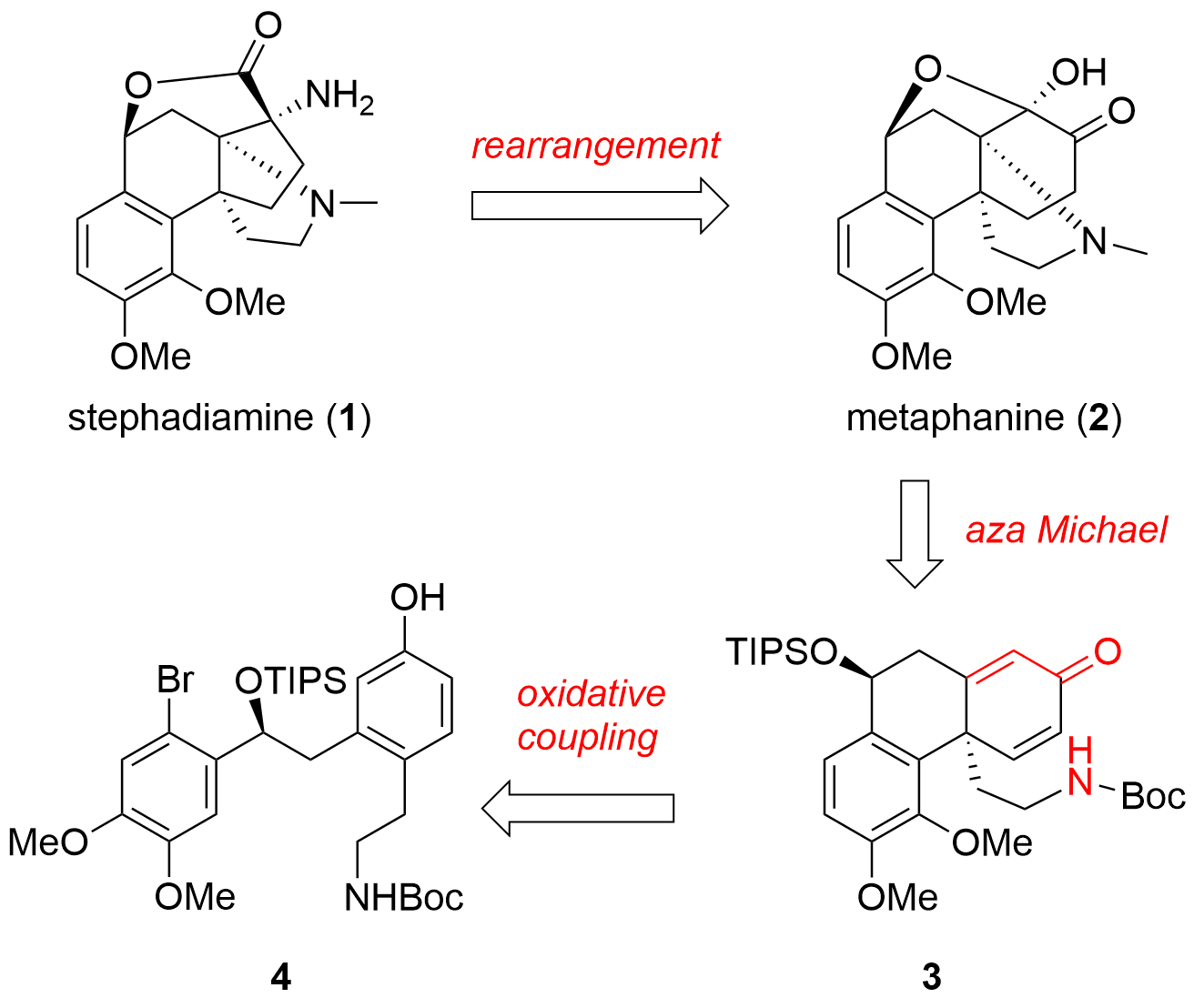 Figure 1. Retrosynthetic analysis for the total synthesis of stephadiamine (1) and metaphanine (2).
Figure 1. Retrosynthetic analysis for the total synthesis of stephadiamine (1) and metaphanine (2).
Oxidative coupling towards fragment 3
For the synthesis of fragment 3, first the both fragments 5 and 6 were coupled under basic conditions by deprotonating of 5 as shown in Scheme 1. The cyanohydrine 5 itself can be synthesized in one step from literature-known aldehyde, mesylate 6 was synthesized in 5 steps. After coupling, racemic alcohol 7 was liberated by deprotection and reduction. Through kinetic resolution the racemic substrate could be transferred into 8 with high enantioselectivity. The secondary alcohol was protected, and the terminal alkene was decomposed to the primary alcohol 9 by ozonolysis. The alcohol was converted into the protected amine in a three-step procedure and MOM ether was deprotected to synthesize the cyclization precursor 4. The key reaction, an oxidative coupling, could be induced with PIDA and led to 10 as a single isomer, however with low yield.
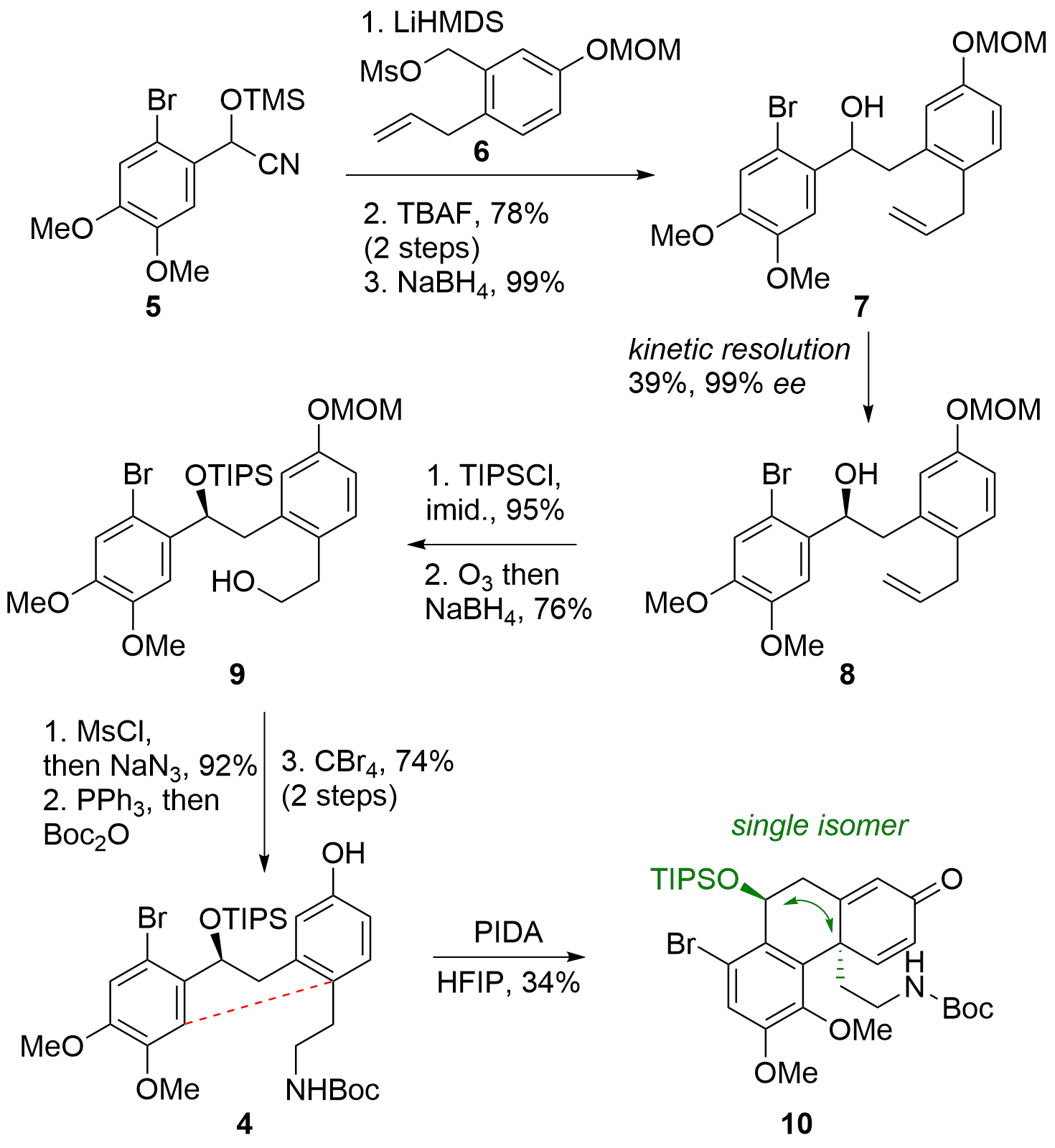 Scheme 1. Synthesis of fragment 4 and oxidative coupling to 10.
Scheme 1. Synthesis of fragment 4 and oxidative coupling to 10.
Completion of the total synthesis
After the oxidative coupling the synthesis of the 1 and 2 could be realized in a straightforward way by the Nagasawa group as shown in Scheme 2 and 3. In detail, debromination delivered 3 in excellent yield. In the next step, for the regioselectivity (7.3:1) of the aza Michael reaction it was crucial to add HMPA as co-solvent. The resulting ketone 11 was oxidized in α position in two steps to the diketone 12. Finally, silyl deprotection in situ to the hemiketal and reduction gave 13 in both good yields. In the last steps the Boc protected amine was altered into the desired tertiary amine of metaphanine 2.
Scheme 2. Completion of the total synthesis of metaphanine (2).
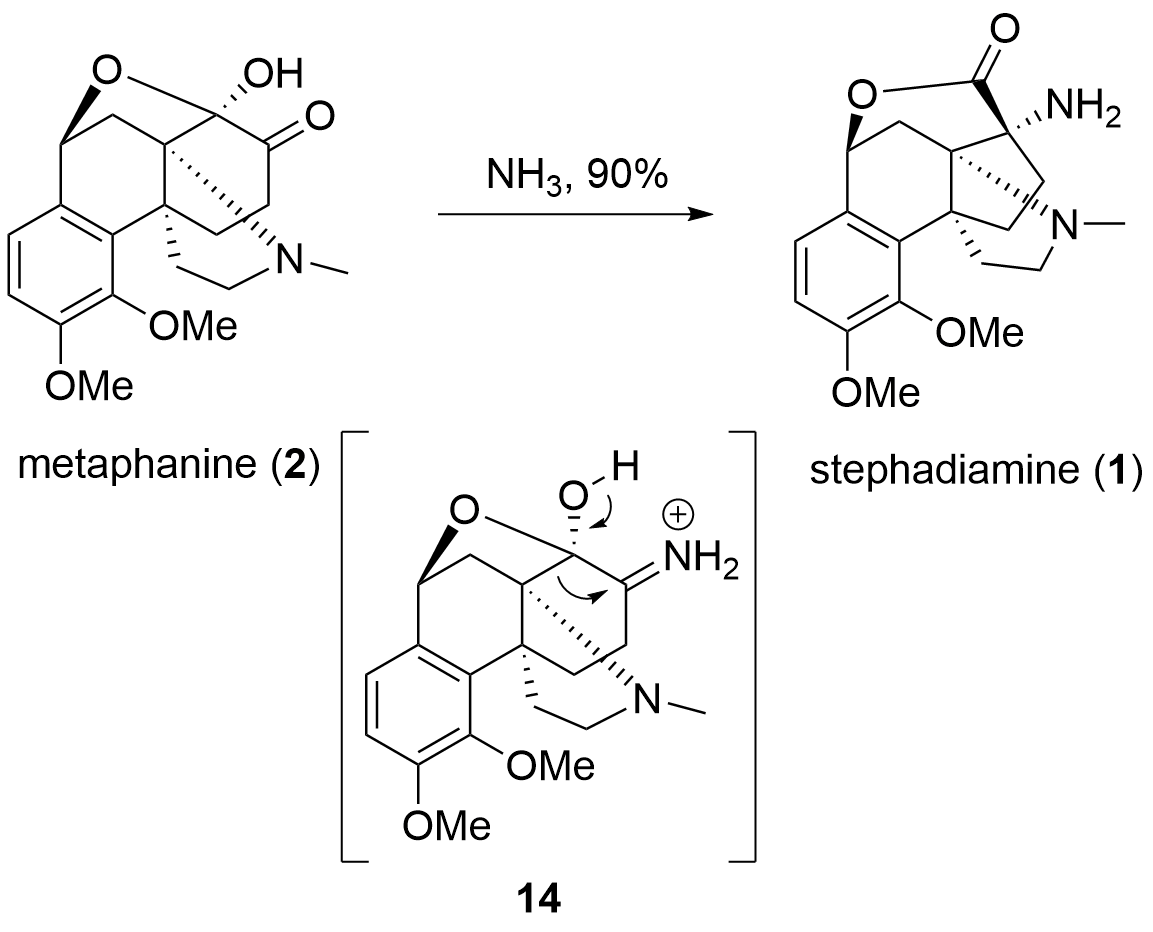
Scheme 3. Conversion of metaphanine 2 to stephadiamine 1 by ammonia.
In total, the Nagasawa group was not only able to successfully synthesize metaphanine 2 in 23 steps, but also to demonstrate a possible biosynthetic relationship between the two natural products 1 and 2. The key-reactions of this synthesis were the intramolecular, stereoselective oxidative coupling and the regioselective aza Michael reaction.
Published in: M. Odagi, T. Matoba, K. Hosoya, K. Nagasawa J. Am. Chem. Soc. 2021, 143, 2699-2704. doi: 10.1021/jacs.1c00047.