Total Synthesis of Gibberellin GA18 Methyl Ester (M. Dai, 2022)
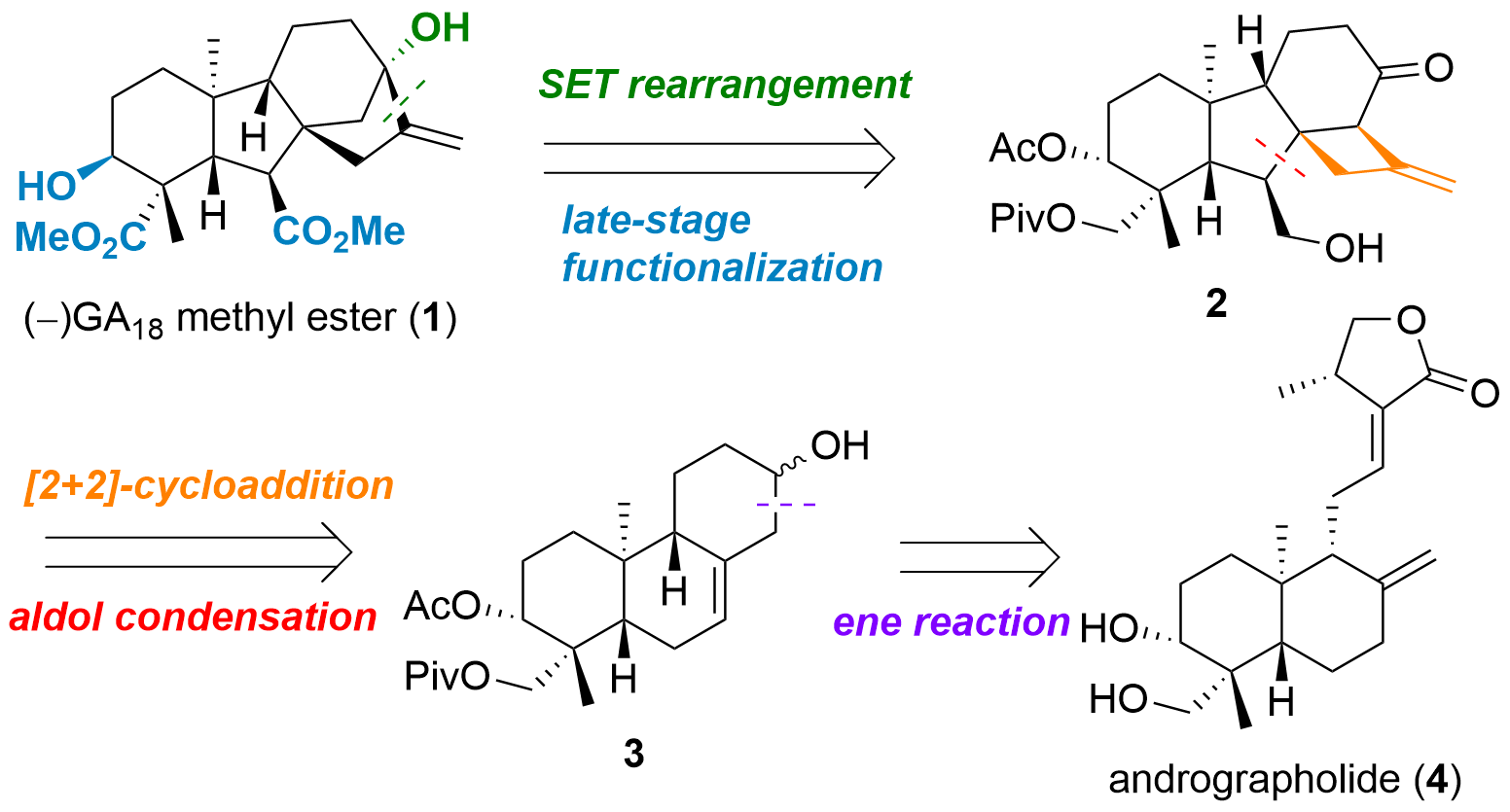
Gibberellins are plant hormones that are synthesised in a variety of ways before. In the paper published in the Journal of American Chemical Society (10.1021/jacs.2c12470), the research team of Mingji Dai from Emory University (USA) targeted GA18 methyl ester (1, Figure 1), a tetracyclic terpenoid. They planned to synthesise 1 from cyclobutane 2 by late oxidation steps and a single electron transfer (SET) initiated rearrangement. The cyclobutane 2 itself was to be obtained from 3 by cycloaddition and aldol condensation. Finally, 3 should be synthesised from andrographolide (4).
Figure 1: Structure of GA18 methyl ester (1) and retrosynthetic analysis by Mingji Dai.
Synthesis of Cycloaddition Precursor
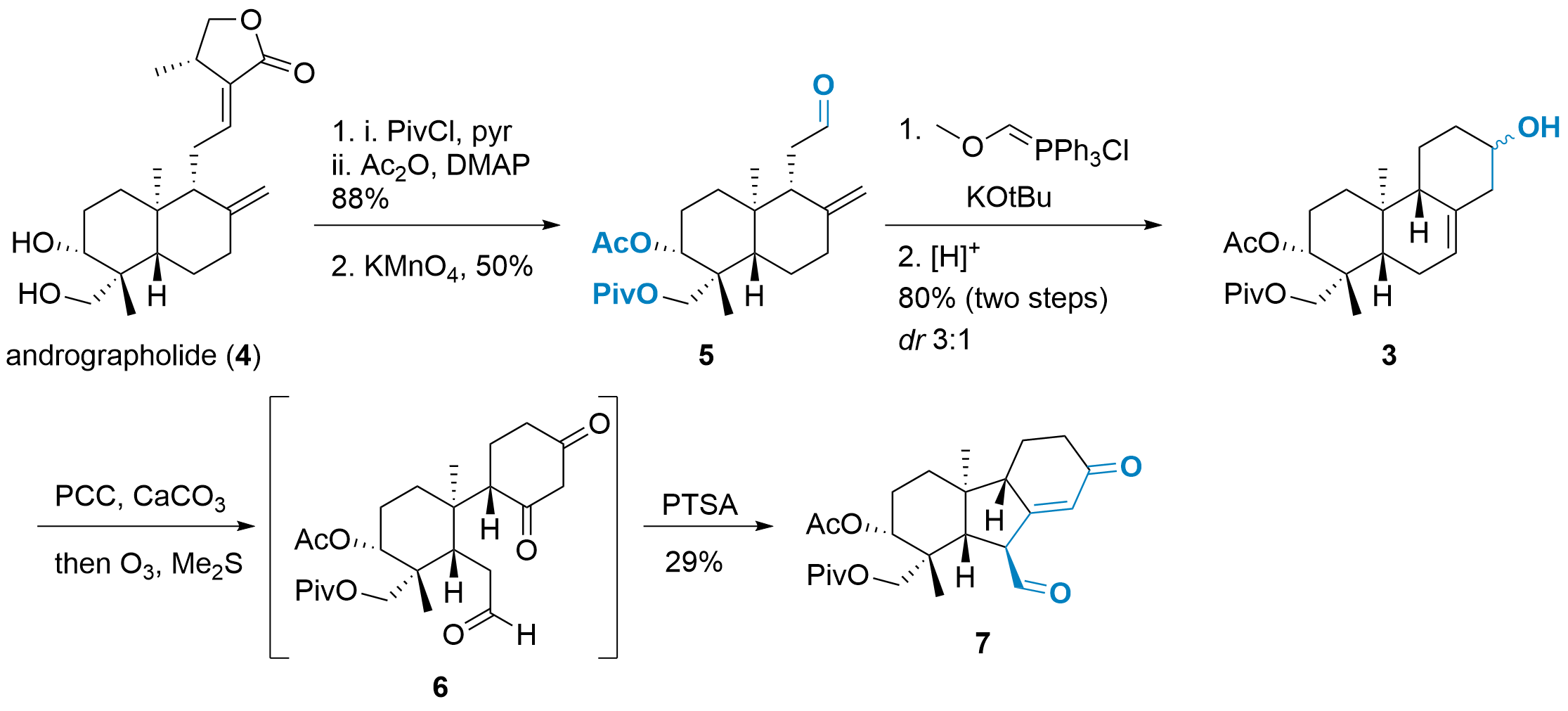
Andrographolide (4) is a cheap starting material, but it requires modifications prior to the assembly of the tetracyclic core, as shown in Scheme 1. Consequently, diprotection of the alcohols was followed by oxidative cleavage to give the aldehyde 5. The Wittig homologation strategy then led to the extended aldehyde, which reacts in situ by an ene reaction yielding 3. Since the alcohol is oxidized in the next step, the diastereoselectivity of this reaction did not play a major role. The oxidation was followed by ozonolysis, which led to carbonyl 6. Acid treatment then initiated the aldol condensation to 7.
Scheme 1: Synthesis of intermediate 7 from andrographolide (4).
Cycloaddition and SET Rearrangement
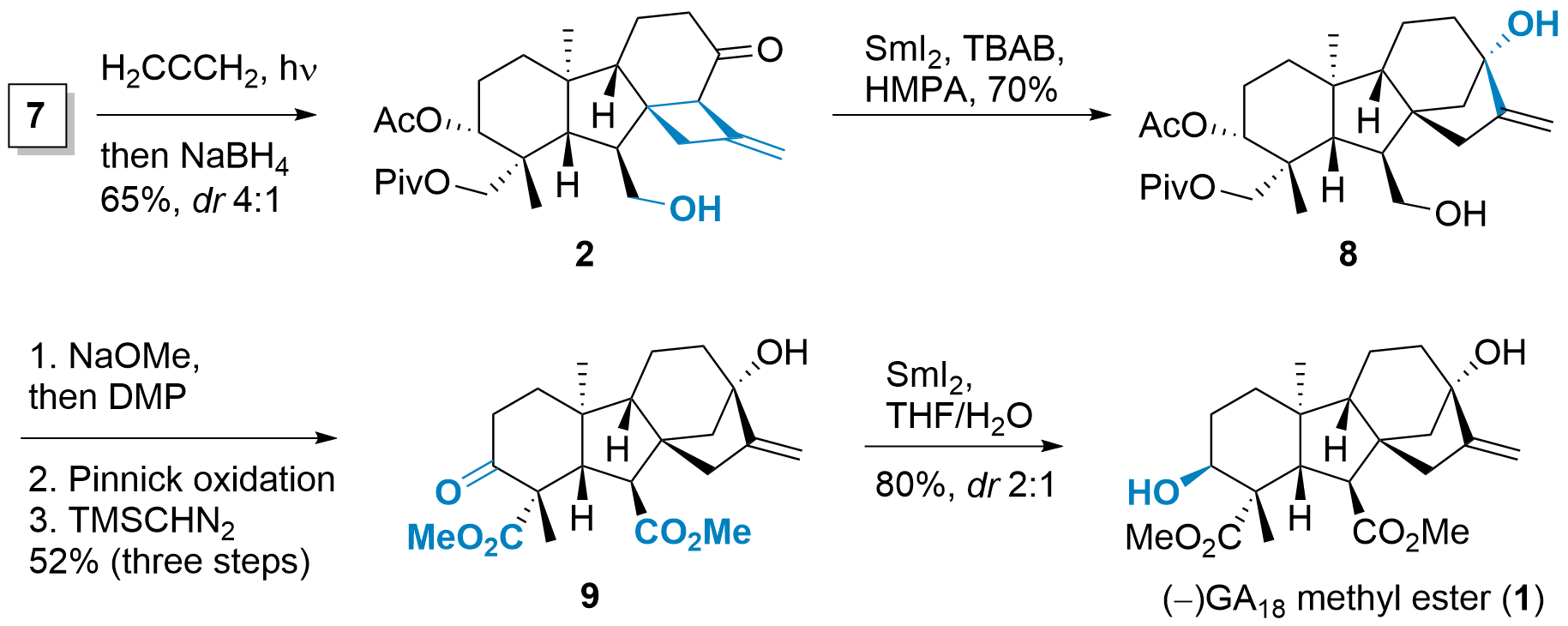
With 7 in hand, the [2+2] cycloaddition was probed, which gave 2 after reduction in accetable dr, as shown in Scheme 2. The authors noted that the cycloaddition with reduced 7 gave lower diastereoselectivity. Subsequently, the tetracyclic core was constructed by single electron transfer initiated rearrangement. For this, a carbonyl radical was generated by SmI2 and transferred to the designated alcohol 8 by rearrangement. From here, 1 could be synthesised in a straightforward manner. Removal of all protecting groups was followed by oxidation and methyl ester formation to give 9. The authors then found that the reduction of 9 to 1 was sluggish and gave mainly the wrong isomer using standard conditions (e.g. NaBH4). Only under unusual radical conditions 1 could be synthesized with low diastereoselectivity. However, the epimer (not shown) could be oxidised back to 9.
Scheme 2: Finalization of the total synthesis of 1.
In this study, Dai et al. were able to successfully report a short synthesis of 1. They were able to construct the tetracyclic core in a rapid synthesis and were able to avoid unnecessary purification steps. The use of andrographolide (4) as a cheap starting material is appealing and could theoretically lead to better accessibility of 1. Unfortunately, the final reduction seems to be unselective, resulting in only milligram amounts of 1. The work nevertheless provides an invaluable insight into the synthesis of this important plant hormone.
Published in: Lei Li, Weida Liang, Mario E. Rivera, Ye-Cheng Wang, Mingji Dai Journal of American Chemical Society 2022, 10.1021/jacs.2c12470.

No Comments