Total Synthesis of racemic Phyllantidine by Ring Expansion (J. L. Wood, 2020)
Phyllantidine (1) is member of the Securinega alkaloids family named after their first isolation from Securinega plants. Nowadays over 50 members are known. Both enantiomers could be isolated in the past and one stereoselective total synthesis by Michael Kerr is described in literature. As shown in Figure 1 especially the embedded nitrogen oxygen bond remains challenging for synthetic approaches to the oxazabicyclo[3.3.1]nonane. Now the research group of Prof. John L. Wood from Baylor University (USA) in cooperation with Prof. K. Wiberg from Yale University (USA) published in the journal Angewandte Chemie an innovative synthesis of racemic phyllantidine (1) featuring a ring expansion strategy to the crucial N-O bond. From a retrosynthetic point of view the butyrolactone moiety was introduced by a late-stage butenolide formation from 2, which itself is derived from 3 by iodo cyclization. Hydroxamic acid was synthesized from aldol product 4 by ring expansion - the key reaction of the synthesis.
Figure 1: Retrosynthetic analysis for the total synthesis of phyllantidine (1).
Synthesis of acyloxy nitroso key fragment
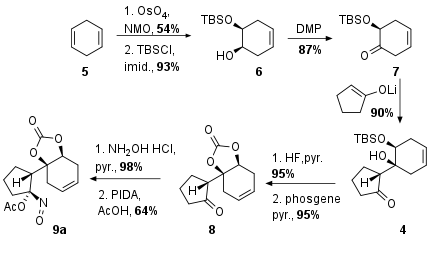
As shown in Scheme 1 the Wood research group decided to start with diene 5, which delivered monoprotected diol 6 after syn selective Upjohn dihydroxylation with highly toxic OsO4 and TBS protection. The unprotected alcohol was then transformed into the ketone 7 by DMP oxidation. The following aldol addition yielded in 4 as the only isomer in excellent 90% yield. Fluoride mediated silyl deprotection (95%) was followed by carbamate formation with toxic phosgene (95%) to the bicyclus 8. Finally, oxime formation was followed by oxidation to the acyloxy nitroso substrate 9a.
Scheme 1: Synthesis of key fragment 9a.
Ring Expansion and Completion of Total Synthesis
The following ring expansion, which was the first time used in a complex total synthesis, was extensively explored and supported by computational investigations. Using substrates 9b-c led to a mixture of regioisomers with the preference of undesired isomer of the type 10 (see Scheme 2). After studying solvent effects, the effect of the counter anion and reaction conditions, hydroxamic acid 3 could be synthesized in 62% yield as single isomer under optimized conditions. Iodocyclization led to substrate 11 in excellent yield. DBU mediated iodide elimination was followed by challenging carbamate cleavage to diol 12. DMP oxidation and treatment with the Bestmann ylide finally liberated the natural product 1 as a racemic mixture.
Scheme 2: Ring-expansion and completion of total synthesis of phyllantidine (1).
In this study the Wood research group could successfully implement a new approach to the embedded N-O bond moiety by ring-expansion from hydroamic acids. Interestingly the initial regioselectivity could be reversed by the combination of computational and experimental chemistry. In the end the alkaloid 1 was synthesized in 14 steps from commercially available material.
Published in K. M. Lambert, J. B. Cox, L. Liu, A. C. Jackson, S. Yruegas, K. B. Wiberg, J. L. Wood Angewandte Chemie Int. Ed. 2020, 59, 9757-9766. doi: 10.1002/anie.202003829
For another synthesis by John L. Wood, see the total synthesis of alterbrassicicene C in 2022.



No Comments