Total Synthesis of Salimabromide (Hai-Hua Lu, 2022)
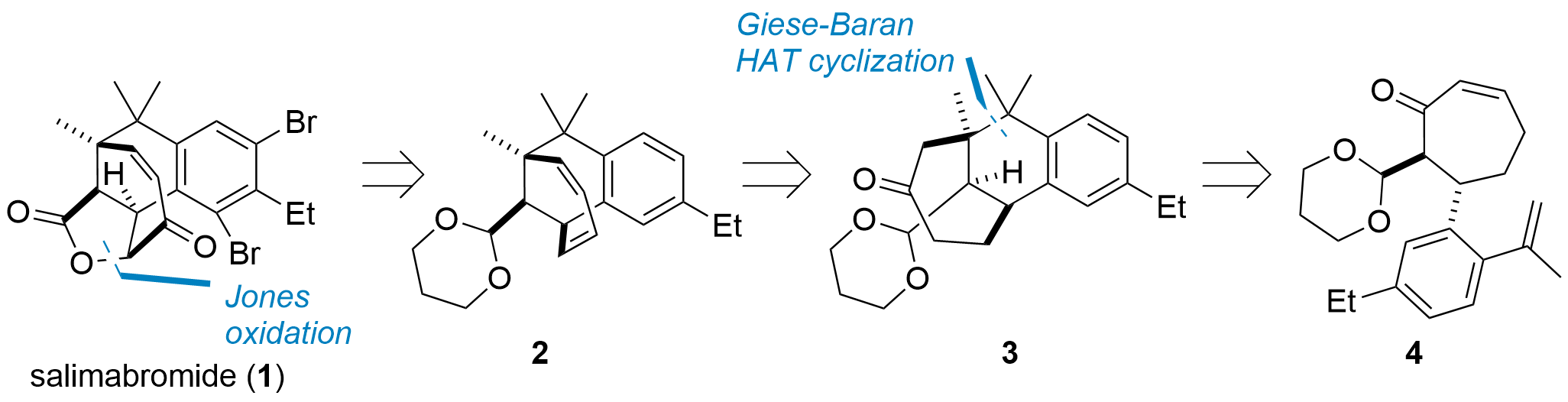
Salimabromide (1, Figure 1) is a natural product of marine source with an unique structure. Since the isolation 2013 two total synthesis are reported by Thomas Magauer (JACS 2018: 10.1021/jacs.8b06228) and Dirk Menche (Org. Lett. 2019: 10.1021/acs.orglett.9b00706). The research group from Hai-Hua Lu from Westlake University (Hangzhou, China) was now able to report a novel synthetic route in the Journal of American Chemical Society (JACS 2022: 10.1021/jacs.2c08337). Their novel approach features a short and concise synthesis in only 8 steps from commercially available starting materials. From a retrosynthetic point of view, salimabromide should be available from diene 2 by oxidation, which itself is availble from ketone 3. In their key step, this tricyclic core should be available from 4 by a HAT promoted Giese addition.
Figure 1: Retrosynthetic analysis of salimabromide by H. Lu et al.
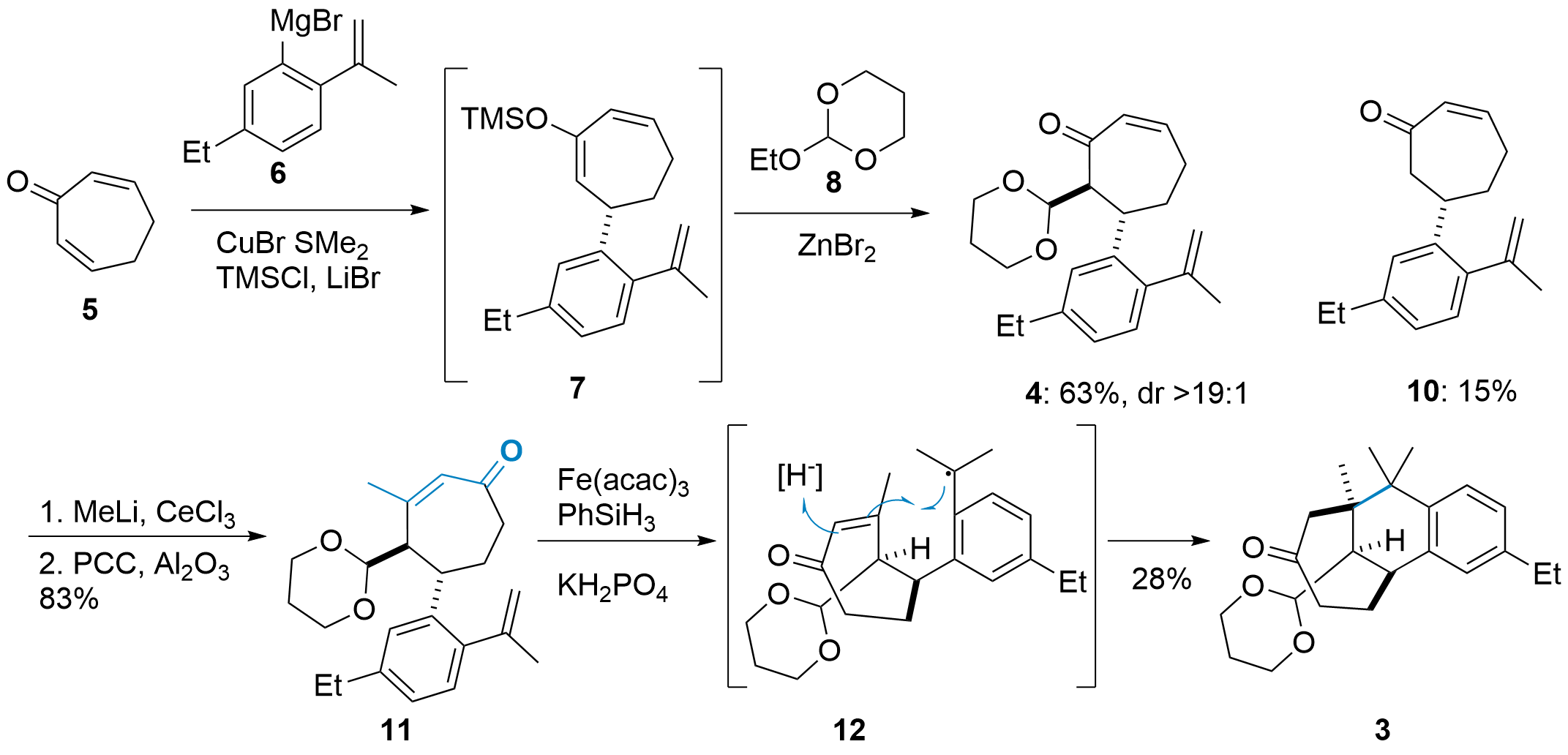
To synthesize enone 4 the Lu group started with heptdienone 5 and Grignard reagent 6 as shown in Scheme 1. After copper mediated 1,4-addition, the enolate is trapped by TMSCl and the following diastereoselektive addition of 8 was mediated by Lewis acid. Beside the product 4 minor amounts of quenched enolate 10 was isolated, however, the authors could report a single step transformation of 10 into 4. Next, methylation of the ketone was followed by oxidative rearrangement (Babler-Dauben oxidation) to key intermediate 11. Hydrogen atom transfer (HAT) was initiated by Fe(acac)3 using PhSiH3 as stochiometric reductant. Note the low yield of 3, which is the result of a side product from an unproductive [1,5] HAT (not shown).
Scheme 1: Synthesis of key intermediate 11 and Giese type cyclization to 3.
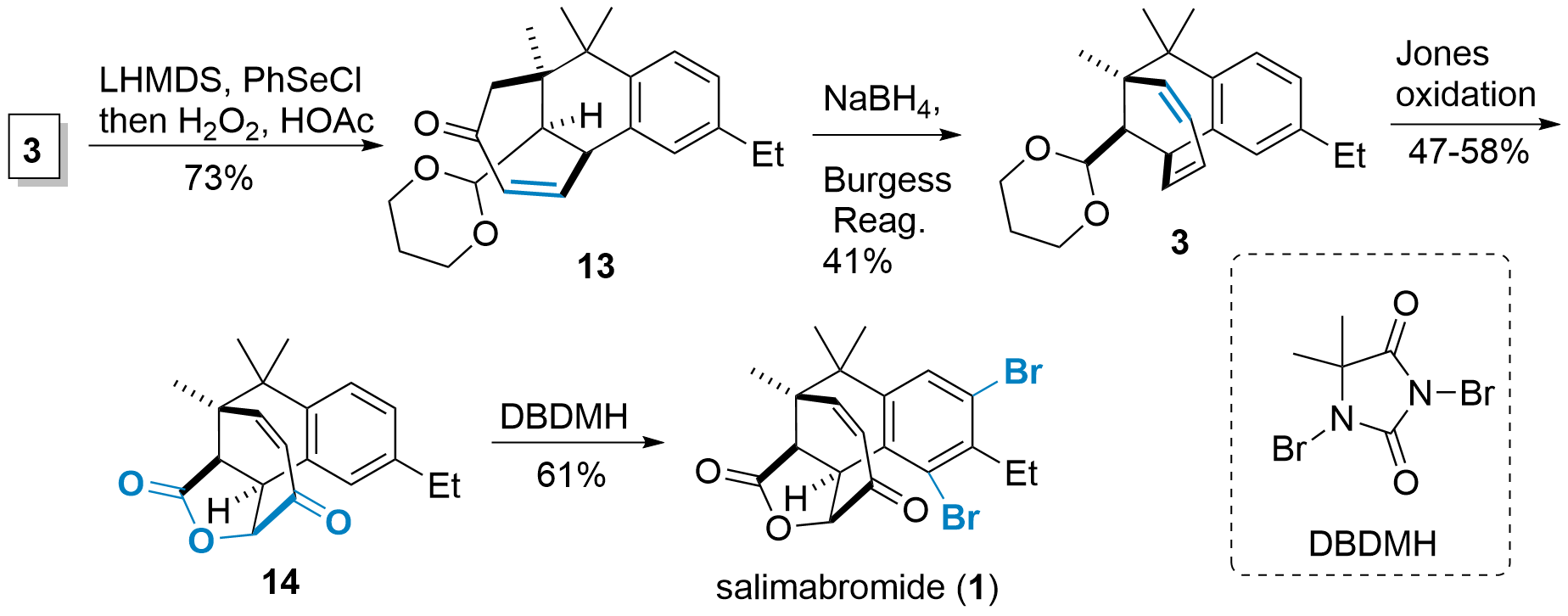
Finally, the synthesis of salimbromide from 3 was finished in 4 steps as shown in Scheme 2. In detail, formation of enone by dehydrogenation led to 13. The ketone was then reduced with sodium borohydride and elimination with Burgess reagent led to diene 3. Jones oxidation under acidic conditions liberated the aldehyde, which directly was oxidized to the acid. The acid then reacted with the diene and final oxication gave 14. Dibromation using DBDMH finally led to the natural product as a racemic mixture.
Scheme 2: Completion of total synthesis of salimabromide (1).
In conclusion, Lu and coworkers succesfully reported a very short route to salimabromide and were able to synthesize more than 30 mg of the rare natural product. Although the natural product itself is nearly racemic (see Menche: 10.1021/acs.orglett.9b00706) they also could provide a stereoselective access to 10.
Published in: Hai-Hua Lu, Kang-Ji Gan, Fu-Qiang Ni, Zhihan Zhang, Yao Zhu Journal of American Chemical Society 2022, 144, 4118778–18783. doi: 10.1021/jacs.2c08337

No Comments